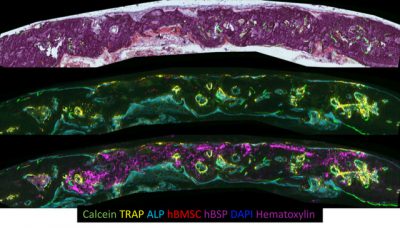
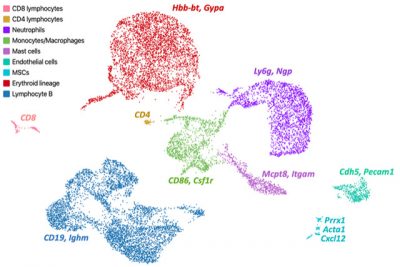
Center Overview
The Center for Regenerative Medicine and Skeletal Development is an interdisciplinary group of basic scientists engaged in advancing understanding of the biology of bone, craniofacial development, cartilage, and skin, and exploring the potential for regeneration of these tissues lost due to trauma, disease and aging. The Center was formed in 2005 with an National Institutes of Health (NIH) infrastructure award to the UConn School of Dental Medicine, in alignment with the university’s strategic plan to build on existing extraordinary strengths in the area of musculoskeletal biology.
Areas of research within the Center encompass skeletal stem/progenitor biology, cartilage and skin differentiation, craniofacial genetics, and temporomandibular joint remodeling and disease, and the use of stem cells for skeletal tissue regeneration and repair.
The Center offers research education and training opportunities for postdoctoral fellows, graduate students, and Ph.D., D.M.D., and M.D. degree students, in part through institutionally supported opportunities including the NIH/NIDCR funded T90 training grant.
In addition to directing independent research programs, integrated research efforts among Center faculty have led major endeavors including the State of Connecticut stem cell group and bone phenotyping knockout mouse project (KOMP). The Center is committed to multidisciplinary research collaborations within and outside UConn Health and is aligned with the UConn Musculoskeletal Institute and the Department of Biomedical research.
The Center consists of approximately 40 individuals including the director, faculty, fellows, graduate students and staff, supported primarily by NIH funding. The 12 independent investigator laboratories of the center occupy adjacent space on two research floors, and include dedicated space for fluorescent microscopic imaging and histological tissue preparation/analysis and two surgical suites for generation of numerous bone injury/regeneration models.
The Center is well equipped with shared instrumentation that includes Kubtek-X-ray imaging system for small animals, two Zeiss Axioscan fluorescent scanning microscopes, molecular devices IXM confocal microscope, Zeiss Axio-Observer, Olympus IX50 inverted fluorescent and three cryostats.
Academic Programs
For information about education and research training with the Center for Regenerative Medicine and Skeletal Development, visit the Reichenberger Lab and the Bonebase Lab.
Faculty
Ivo Kalajzic, M.D., Ph.D.
Center Director
Email: ikalaj@uchc.edu
David W. Rowe, M.D.
Professor
Email: drowe@uchc.edu
Peter F. Maye, Ph.D.
Associate Professor
Email: pmaye@uchc.edu
Ernst J. Reichenberger, Ph.D.
Professor
Email: reichenberger@uchc.edu
Yadav Sumit, B.D.S., M.D.S., Ph.D.
Associate Professor of Orthodontics
Email: syadav@uchc.edu
Elaine Dutra, D.D.S., M.S.D., Ph.D.
Assistant Professor of Orthodontics
Email: edutra@uchc.edu
Dashzeveg Bayarsaihan, Ph.D.
Associate Professor
Email: dashzeveg@uchc.edu
Xiaonan Xin, Ph.D.
Assistant Professor
Email: xin@uchc.edu
Sanja Novak, Ph.D.
Instructor
Email: snovak@uchc.edu
Vanessa Scanlon, Ph.D.
Assistant Professor
Email: scanlon@uchc.edu
I-Ping Chen, D.D.S., Ph.D.
Associate Professor of Endodontics
Email: ipchen@uchc.edu
Emily Germain Lee, M.D.
Professor of Medicine
Email: germainlee@uchc.edu
Contact Information
Dr. Ivo Kalajzic
Center Director
Phone: 860-679-6051
Email: ikalaj@uchc.edu